Anovulation is one of the common causes of infertility.Polycystic ovary syndrome (PCOS) is the most common chronic anovulatory disorder.To our knowledge, insulin resistance is significantly associated with PCOS.Therefore, in patients with PCO, insulin-sensitizing drugs such as pioglitazone can be used to stimulate ovulation.
Sixty-one patients with PCOS were included in the study according to the inclusion/exclusion criteria after obtaining approval from the Ethics Committee of the Medical University of Mashhad.The patients were divided into two groups.The first group took 30 milligrams (mg) of pioglitazone daily starting on the second day of their menstrual period.The second received a placebo.150 mg of clomiphene citrate was administered from day 3 to day 7 of the menstrual cycle.Vaginal ultrasonography was performed on all women, and in cases of mature follicles, intrauterine insemination was performed after injection of human chorionic gonadotropin.Ovarian stimulation and pregnancy rates were compared in each group.
There were no differences between groups in terms of demographic characteristics and types of infertility.Body mass index was higher in the pioglitazone group (28.3 ± 3.8 vs 26.2 ± 3.5, P value = 0.047).Follicle size did not differ significantly between groups (2.2 ± 1.4 vs 1.3 ± 1.1, P value = 0.742).Pregnancy rates [4 (12.9%) vs 4 (13.3%), P value = 1] did not differ between groups.

Despite the higher number of follicles in the pioglitazone group, our study showed no difference in ovarian stimulation and pregnancy rates.
Infertility affects about 10-15% of couples.30% of female infertility is due to ovulation failure [1].Polycystic ovary syndrome (PCOS) is the most obvious and common disorder associated with chronic ovulatory disorders [2].When using the European Society for Human Reproduction and Embryology and American Society for Reproductive Medicine (ESHRE/ASRM) diagnostic criteria, the prevalence of PCOS is approximately 15-20% [3].
Abnormal lipoprotein levels are typical of PCOS patients, with elevated total cholesterol (Chol), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and apoptotic AI [4] , 5,6].The most significant change in lipids reported was a decrease in HDL.Hyperinsulinemia and insulin resistance (IR) are common in PCOS.Mustafa et al.About 46% of Egyptian women with PCOS were found to have IR [4, 7].Insulin disrupts steroidogenesis in the ovary independent of gonadotropin secretion I PCOS [1].Insulin receptors and insulin-like growth factor-1 (IGF-I) are present in ovarian stromal cells [5].Decreased autophosphorylation, a specific disorder associated with insulin receptor-mediated signaling, is detected in 50% of women with PCOS [3].
Abnormal glucose metabolism significantly improves weight loss; weight loss may reduce hyperandrogenism and restore ovulatory function [7].Obese women with insulin resistance, calorie restriction, and weight loss reduce the severity of insulin resistance.On the other hand, a decrease in insulin concentration reduces androgen production [8].
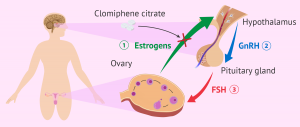
Today, clomiphene citrate is the recommended treatment for ovulation induction in women with PCOS.Insulin resistance is significantly associated with polycystic ovary syndrome, so drugs that increase insulin receptor sensitivity, such as metformin and beta-thiazolidinediones, are considered in the treatment of these patients.Treatment of insulin resistance may induce ovulation, especially in obese women with a higher degree of insulin resistance [9].
Insulin resistance implies a reduced glucose response to insulin, followed by hyperinsulinemia, which leads to elevated triglycerides, reduced HDL-cholesterol, glucose intolerance, and cardiovascular risk [10].Pioglitazone, used to treat type 2 diabetes, directly affects peripheral insulin sensitivity.In some recent studies, pioglitazone has been shown to reduce intra-ovarian stromal blood flow.It may help improve ovarian stimulation and in vitro fertilization (IVF) outcomes in PCOS patients.Coffler showed that pioglitazone can significantly induce ovulation in hyperinsulinemic patients [11].
To date, no studies have examined the effect of pioglitazone on fertility in our patients.Therefore, we hypothesized that pioglitazone as an insulin disinfectant might improve ovulation and pregnancy rates in PCOS patients.This study aimed to use pioglitazone for successful pregnancies, including chemical and clinical pregnancies, and the number of large follicles in infertile women with PCOS.
Mashhad Medical University oversaw this randomized clinical trial study from 2014 to 2017 and used a non-probability sampling method to recruit 61 PCOS patients who were referred to the Milad Infertility Center for infertility treatment.The ethics committee of the Mashhad Medical University approved the moratorium on “March 15, 2014″ and written informed consent was obtained from all participants.
Inclusion criteria were infertile women aged 18-38 years with normal hysterosalpingography and spermogram.The diagnosis of polycystic ovary syndrome is based on the AES criteria (Androgen Excess Society 2006) based on the above criteria: (1) hirsutism or hyperandrogenic symptoms.(2) Ovarian dysfunction is oligomenorrhea, or polycystic ovary is diagnosed as cervical lace-like appearance by ultrasound; (3) The promotion of secondary causes such as ovarian and adrenal tumors and pituitary adenomas.Polycystic ovary syndrome is diagnosed if the menstrual cycle is oligomenorrhoea, or if the number of peripheral follicles in the ovary is 2-9 mm greater than 9 on the Ferriman-Gallway scale.
Patients with a history of chronic cardiovascular disease, chronic kidney disease, diabetes, thyroid disease, and lung disease were excluded.

After selecting eligible patients, they were divided into two groups by simple random sampling using computer software.The envelope method was used to randomly assign patients to study groups.In this way, the random number will be put into a sealed envelope.The contents of the envelope cannot be seen from the outside.Group A contained 30 tablets of pioglitazone, 30 mg, and 15 tablets of clomiphene, while group B was placed with 30 tablets of placebo and 15 tablets of clomiphene.Patients were blinded to the assigned treatment.
All patients underwent transvaginal ultrasonography on the second day of menstruation and were included in the study if there were no ovarian cysts larger than 20 mm.
The number of medium and large follicles and endometrial thickness were assessed on the tenth or eleventh day of menstruation.Chemical and clinical pregnancy rates were assessed.
The first group received 30 mg of pioglitazone daily; the second group received a placebo starting on the second day of menstruation.Between days 3 and 7 of the menstrual cycle, both groups were given 150 mg of clomiphene citrate.Transvaginal ultrasonography on day 10 or 11.Consider human chorionic gonadotropin (HCG) followed by intrauterine insemination (IUI) in women with endometrial thickness greater than 7 mm and follicles greater than 16 mm.
In the case of a 5-day delay in menstruation, blood samples were taken to assess βHCG levels.Pioglitazone-related side effects and follicle numbers greater than 16 mm and endometrial thickness were assessed during the study.Finally, ovarian stimulation and pregnancy rates were compared across groups.
The sample size was calculated using the PASS 11 software and the mean number of follicles in each group was compared.By default, Type 1 errors are 5% and Type 2 errors are 20%.We estimated 22 patients per group, but due to potential attrition, 30 participants per group were considered.
Data were entered into SPSS version 16.Initially, the characteristics of each group were described by descriptive statistical methods, including means and standard deviations for continuous variables and numerical plus frequencies for categorical variables.Then, to compare quantitative variables in the two study groups, independent t-tests or Mann-Whitney-U tests were used after assessing normality using the Kolmogorov-Smirnov test.Qualitative variables were compared using the chi-square test.In all statistics, P-values less than 0.05 were considered significant levels.
Regarding inclusion criteria, 93 women participated in the study, 19 had exclusion criteria and 13 dropped out.Thirty patients were classified in the placebo group and 31 in the intervention group.The CONSORT algorithm is shown in Figure 1.The demographic characteristics of women are shown in Table 1.There were no differences between groups in terms of demographic characteristics and type of infertility.The average age of the intervention group was 28.20±5.46 and that of the control group was 27.07±4.18, and the difference was not statistically significant.However, body mass index (BMI) was higher in the pioglitazone group.
Table 2 summarizes the patient’s sonographic findings, such as the number of medium-sized follicles, the number of large follicles, the maximum follicle size, and endometrial thickness.As shown in Table 2, the size of the follicles was in the group except the medium-sized follicles.
Information on ovulation induction treatment outcomes, such as ovulation volume, chemical, and clinical pregnancy rates per cycle, is presented in Table 3.Ovarian stimulation and pregnancy rates did not differ between groups.
The results of this study showed that there was a significant difference in the number of ovulation stimulations among patients treated with pioglitazone.Ultrasonography, performed on day 10 of menstruation, showed a significant increase in the mean number of follicles in the intervention group.Our findings confirm the findings of a 2012 study on the role of pioglitazone in ovulation induction in hyperinsulinemic patients with PCOS [12].Morley et al.Increased ovulation has also been reported in PCOS patients taking pioglitazone [13].
There were no differences in ovulation and pregnancy rates between the two study groups.This may be due to the duration of pioglitazone used prior to starting clomiphene.Ota demonstrated that 2008 results showed that 7 of 9 patients who took pioglitazone for 12-30 weeks before clomiphene became pregnant [14].Kim’s 2010 study showed a significant reduction in the number of follicles after pioglitazone was given.Furthermore, in his study, the pioglitazone group had a higher clinical pregnancy rate, but this difference was not statistically significant.This finding is in contrast to our results, but can be explained by patient selection criteria, including clomiphene-resistant patients [15].
Ota showed that pioglitazone could improve pregnancy rates in PCOS patients resistant to clomiphene and dexamethasone [14].It seems that PCOS cases with hyperandrogenemia should be selected more carefully.Patients in the Ota program have different levels of hormones, which can affect the outcome of pioglitazone treatment.In our study, hormone levels did not differ significantly before and after the intervention.
In our study, there were no significant differences in the number of large follicles and endometrial thickness between the intervention and control groups.However, there was a significant increase in the number of medium-sized follicles in the intervention group.
In the present study, the intervention group had a higher BMI, which means that this group may be more likely to develop hyperinsulinemia and affect the outcome, although this difference was not statistically significant between the two groups.
None of our patients experienced side effects.There were no statistically significant changes in liver function tests during the study period.
A major limitation of our study was that the study was designed as a case-control project, which resulted in differences in BMI between the two groups.Therefore, the results may be affected by this difference.However, no similar studies of this two-drug regimen have been performed in patients in our region.However, because of pioglitazone’s effect on insulin resistance, it appears that success rates increase if patients receive pioglitazone for a longer period of time before starting the clomiphene diet.Therefore, more research is recommended to determine the best time to use pioglitazone.
Despite the higher number of follicles in the pioglitazone group, our study showed no difference in ovarian stimulation and pregnancy rates between the two groups.
In fact, we have effectively treated specific problems such as infertility, bleeding from uterine dysfunction and hirsutism in the past.Now we have the opportunity (and indeed the responsibility) to provide interventions to prevent or correct some of the metabolic complications of infertility (which can significantly impact overall health as well as quality and quantity of life).
Post time: Mar-30-2022




