· Price & Quotation: FOB Shanghai: Discuss in Person
· Shipment Port: Shanghai, Tianjin,Guangzhou, Qingdao
· MOQ: 300,000 amps
· Payment Terms: T/T, L/C
Product detail
Composition
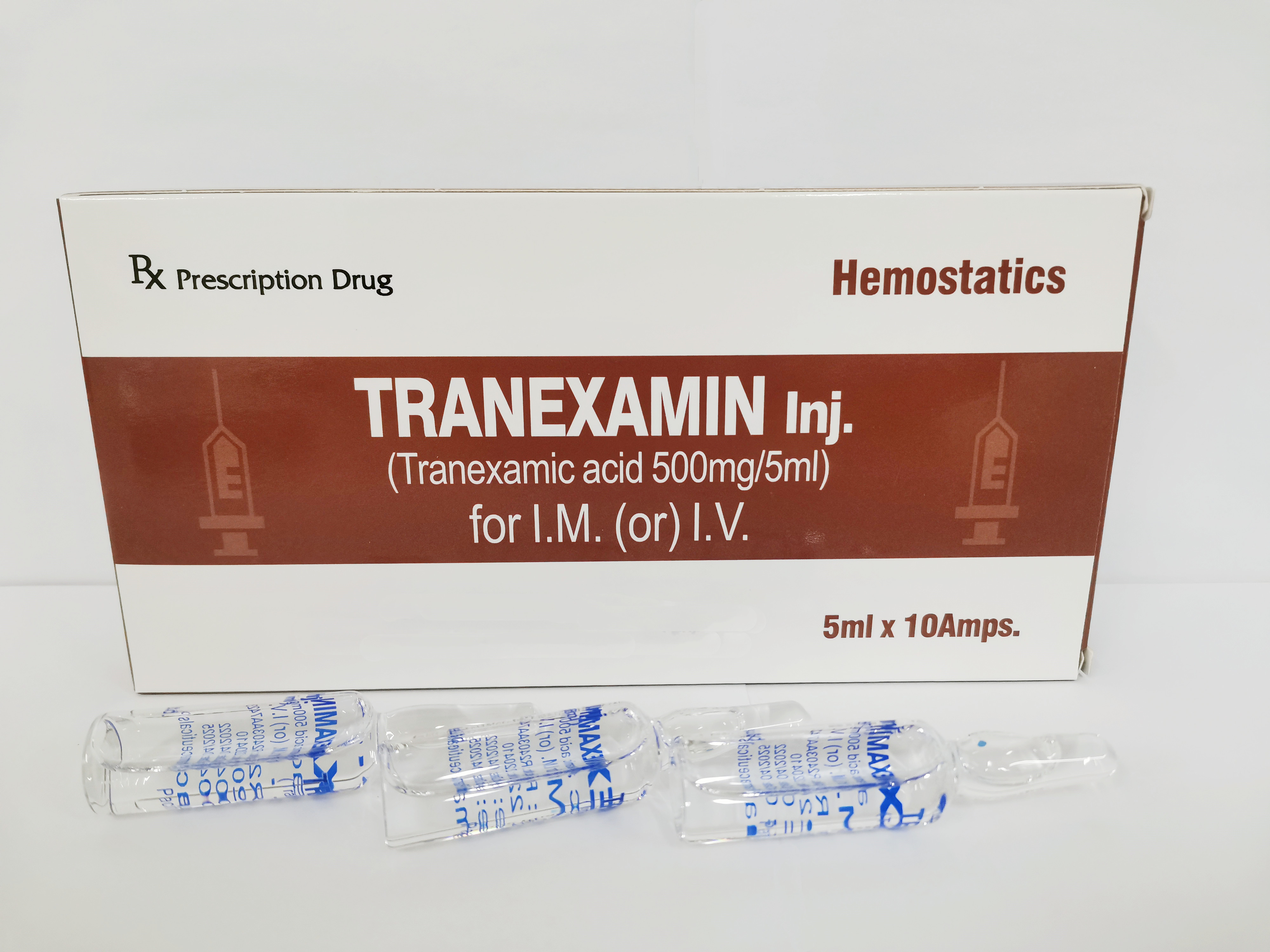
Each ampoule (5ml) contains:
Tranexamic acid 500mg
Description
Colorless and transparent ampoule containing colorless and clear solution
Indications
1.hemorrhagic complications in association with general fibrinolysis (leukemia, purpura, aplastic anemia, per-and post-operative hemorrhage)
2.Hemorrhagic complications in association with local fibrinolysis (pneumorrhagia, nephrorrhagia, prostatectomy, pre-and post-operative hemorrhage)
Dosage & Administration
Tranexamin Inj. 500mg should be administered intravenously by slow injection (If the injection is given too quickly, rarely nausea, chest pain, palpitation, decreasing the blood pressure, dizziness may occur.)
Adult
Administer 250~500mg as tranexamic acid per day in one or two divided I.V or I.M.
If necessary, such as bleeding with intra/post-operative, administer 500~1000mg by IV or 500~2500mg by IV instillation per once.
Dosage should be properly adjusted by ages or symptoms.
Precautions
1.Warning
Injectable ampoule can cause side effects due to glass fraction mixed.
You should carefully cut the ampoule, especially carefully use for the children and elderly.
2.Should not administer to following patients.
1)Patients receiving thrombin.
2)Patients with a history or risk of thrombosis.
3)Patients with hypersensitivity to tranexamic acid or any of the ingredients.
4)Tranexamin injection should not be added to blood for transfusion, or to injections containing penicillin.
3.Should administer with great care to following patients.
1)Patients with thrombus(cerebral thrombosis, myocardial infarction, thrombophlebitis) or risk of thrombosis (there is concerns to be stable thrombus)
2)Patients with consumption coagulopathy (should be concomitantly use with heparin)(there is concerns to be stable thrombus).
3)Patients lying in bed after surgery or patients with pressure hemostasis treatment(increasing the risk of thrombosis because it is easy to occur vein thrombosis. Pulmonary thromboembolism have been reported when moving the bed or stopping the pressure.)
4)Patient with renal insufficiency, because of the risk of accumulation.
5)In patients with disseminated intravascular coagulation (DIC) treatment must be restricted to those in whom there is predominant activation of the fibrinolytic system with acute severe bleeding. Tranexamin Inj. 500mg must not be administration in DIC with predominant activation of the coagulation system. Administration of Tranexamin Inj. 500mg in DIC should be considered only when appropriate haematological laboratory facilities and expertise are available.
4.Adverse reactions
1)Shock: shock may occur rarely, should be monitored fully. If abnormal symptoms occur, stop administering immediately and do proper treatments.
2)Hypersensitivity: rarely hypersensitivity such as pruritus, rash or flushing may occur. In this case, stop administering immediately and do proper treatments.
3)Eyes: temporary disturbance of color vision may occur.
4)Urinary system: urinary tract obstructions in patient with large bleeding (especially hemophilia) from upper urinary tract have been reported.
5)Gastrointestinal system: nausea, vomiting, diarrhea may occur but disappear when the dose is reduced.
6)Others: rarely drowsiness, headache may occur. Rarely thrombosis have been reported.
5.Drug interaction
1)Thrombin: thrombus formation is increased when concomitantly use with thrombin because it promotes the thrombus formation.
2)Thrombosis may occur when concomitantly use with the drugs with actions on hemostasis or hemocoagulase, should be given with caution.
3)Thrombosis may occur when concomitantly use with Batroxobin, should be given with caution.
4)It may enhance the coagulation on strong fibrinolytic activity site such as mouth when concomitantly use with coagulation factor such as Eptacog alfa. Should be given with caution.
6.Pregnancy and Nursing mothers
1)Although there is no evidence from animal studies of a teratogenic effect, should be given with caution because Tranexamic acid crosses the placenta.
2)Tranexamic acid passes over into the breast milk during lactation in concentrations 1/100 of the corresponding serum levels. However, it is considered that no antifibrinolytic effects on the infant.
7.Geriatric precautions
Generally, the aged have poor physiological functions. Should administer with cautions such as decreasing the dose of this drug.
8.Effects on ability to drive and use machines
Not known
9.Overdosage
No cases of overdosage have been reported. Symptoms may be nausea, vomiting, orthostatic hypotension. Maintain a high fluid intake to promote renal excretion.
10.Others
1)In the dog, retina changes have been observed after long-term administration of large doses of tranexamic acid.
2)Compatibility have been reported when concomitantly use with the following solutions; reserpine, tannic phenetidine, concomitant use of methyltestosterone and ethylestradiol, lipid thromboplastin, tissue thromboplastin, sulfadimethoxine.
3)Thrombus formation have been reported when concomitantly large use with the drugs with actions on hemostasis (lipid thromboplastin) by intravenously.
Storage
Hermetic container, store at room temperature (1~30℃)
Shelf life
36 months after manufacturing date
Presentation
5ml × 10 Ampoules / Box